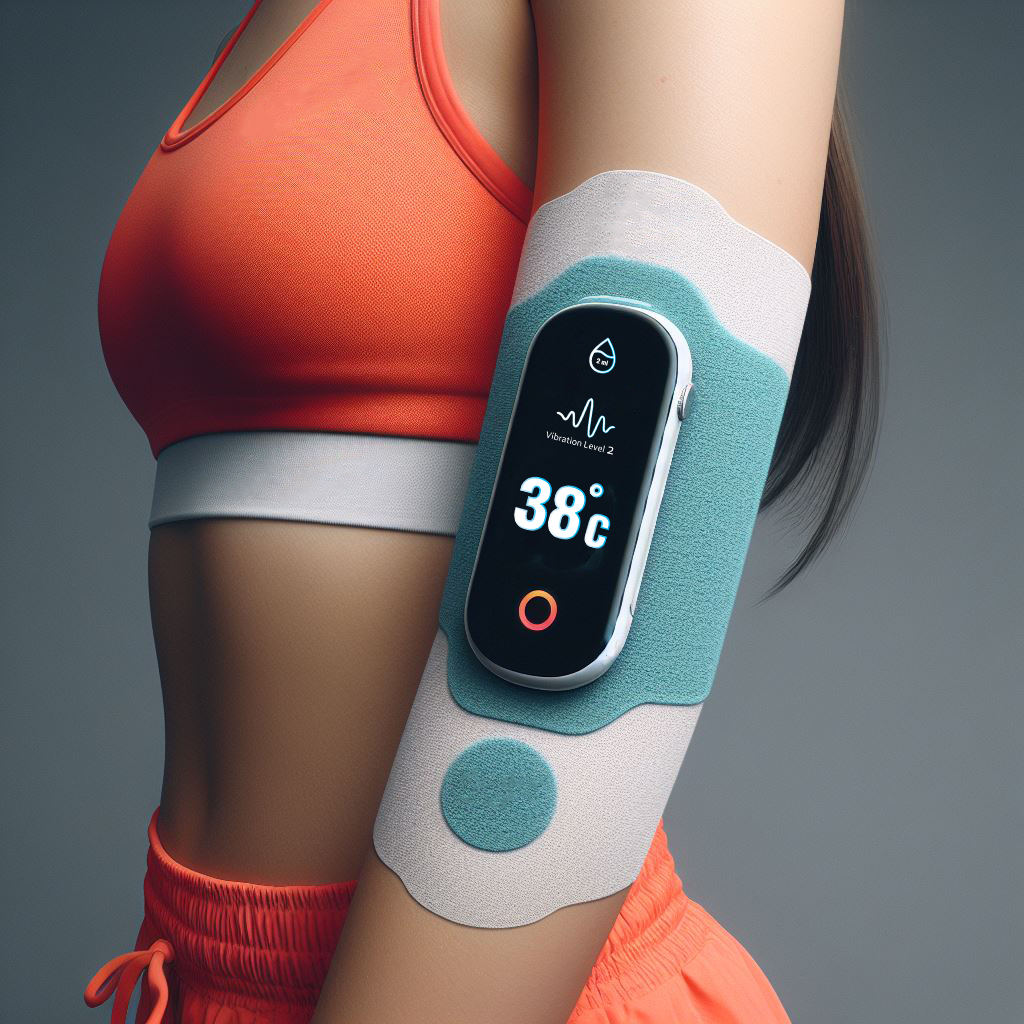
Femto is committed to developing and manufacturing top-tier, AI-enhanced women's wellness devices and services, ensuring quality, effectiveness, and affordability in everything we do

the future of femtech
We aim to enhance women's lifestyles and wellness through cutting-edge technology.
Our proprietary Slow-Release Tech is designed to embed oil and organic botanical substances for multifaceted purposes. By harnessing the power of innovation, we aim to transform daily living for women worldwide.
femto core technology
The Company's SRS (Smart Release System™) proprietary technology utilizes advanced sensors to precisely detect, infuse, and personalize wellness substances, aiming to regenerate women's physical and emotional balance


femto products
Femto's product division focuses on three major anchors: Femto Intimacy, harnessing the company's SRS (Smart Release System) proprietary technology to develop and utilize advanced sensors that precisely detect, infuse, and personalize wellness substances, aiming to support intimacy and well-being. Femto Sports focuses on enhancing well-being experiences pre and post sports activities. Femto Cosmetics concentrates on hair and facial aesthetics
femto analytics
Harnessing the power of big data and artificial intelligence to provide unparalleled, data-driven insights regarding women's wellness. FEMTO owns and markets a proprietary Customer Relationship Management (CRM) software product known as 'Benefit CRM,' which enables small and medium-sized businesses to optimize their day-to-day business activities.

femto CRM











femto team

Yftah Ben Yaackov
CEO and Director

Dr. Stefánia Szabó
President & Director

Avner Tal
VP Sales & Marketing and CTO

Carmel Zigdon
Director

Gabi Kabazo
CFO and Director
INVESTORS RELATIONS
GWI predicts that the wellness economy will return to its robust growth, projecting an average annual growth of 8.6%, with the wellness economy expected to reach $8.5 trillion by 2027. The wellness economy represented 5.6% of global economic output in 2022.*

Disclaimer
This website contains forward-looking statements involving risks and uncertainties that may cause actual results to differ materially from the statements made. Such statements reflect our current views on future events and are subject to such risks and uncertainties. Many factors may cause our actual results to differ materially from those presented on the Site, including those discussed in our filings with Canadian or U.S. securities regulatory authorities. If one or more of these risks and uncertainties, such as currency fluctuations and interest rates, increased competition, and general economic and market factors, occur, or if the assumptions underlying the forward-looking statements prove to be erroneous, Actual results may differ materially from those described herein as intended, planned, predicted or expected. We do not intend and assume no obligation to update these forward-looking statements, except as required by law. The company intends to obtain approval for the EZ-G patent within the usual timetables andto complete the development of the device within the coming year. Additional regulatory standards may be required, including FDA approval or any other approval for manufacturing, marketing and selling the device or capsules under therapeutic indications. There is no certainty that the foregoing approvals will be accepted and all information hereunder is forward-looking. The model of the device appearingon the websiteis not a working device and is a simulation as a concept model only and there is no certainty that it will be compatible with the prototype of When the product is manufactured in the future, there is also no certainty that the oils supplied with the device will belong to Foria, and the matter is subject to the execution of the agreements between the companies.Registration to the site is free of charge and ordering the actual device will require monetary consideration as presented by the company below. The Company reserves the right to terminate the pre-registration process at any time and does not guarantee the timetables for the supply of the products and/or that the product will eventually be supplied.